Degradable zinc sulfide bioorthogonal nanozymes efficiently activate therapeutic molecules
Bioorthogonal chemistry enables unnatural chemical reactions to occur without affecting normal cellular processes. For example, transition metal small molecule catalysts can catalyze chemical reactions in cells that cannot be achieved by natural enzymes, and continuously activate diagnostic and therapeutic molecules (imaging molecules and therapeutic drugs) in situ. However, the direct use of transition metal catalysts faces many problems, including poor water solubility, poor stability, and poor biocompatibility. Transition metal catalysts are very sensitive to serum proteins, which also limits their biomedical applications. Bioorthogonal nanozymes are a new concept proposed in recent years. Loading transition metal catalysts onto the surface of nanoparticles can improve their water solubility, stability and biocompatibility, and also avoid direct contact between transition metal catalysts and proteins. However, most of the carriers of these nanozymes are non-degradable nanomaterials and cannot be excreted from the body, causing long-term toxicity. In addition, improving the catalytic activity of bioorthogonal nanozymes is also a major challenge. Highly active nanozymes can reduce the dosage and potential toxic side effects.
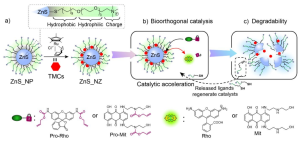
Design ideas
In order to solve the above problems, the author of this article loaded a ruthenium catalyst in the single-molecule ligand layer on the surface of ZnS nanoparticles and constructed a degradable bioorthogonal nanozyme ZnS_NZ, which is used to catalyze the uncaging reaction of diagnostic and therapeutic precursor molecules and realize the realization of therapeutic molecules. activation (Figure 1). The surface of ZnSis modified with a single layer of ligands. One end is a sulfhydryl group and the other end is a quaternary amine group for enhancing water solubility and cellular uptake. The middle contains a hydrophobic carbon chain for stabilizing the catalyst and a hydrophilic polyethylene glycol. . The ZnS core of ZnScan be gradually degraded in cells, and the released surface ligands contain sulfhydryl groups, which can be used as nucleophiles to increase the reaction rate of the rate-determining step of the uncaging reaction. The authors carefully studied the degradation and catalytic enhancement mechanism of ZnSand applied it to the efficient activation of therapeutic molecules at the living cell level.
Figure 1. Structural design of degradable ZnSand its catalytic activation principle
Data introduction
The authors first synthesized ZnS nanoparticles (ZnS_NP) and gold nanoparticles (Au_NP) with a diameter of 15 nm, and compared their degradation abilities. The fluorescence emission spectrum of ZnS_NP decreased by about 20% after five days (Fig. 2a), while the UV-visible absorption spectrum of Au_NP did not change significantly (Fig. 2b). Dynamic light scattering results show that the hydrated particle size distribution of ZnS_NP has a smaller particle size peak (Fig. 2c), while there is no obvious change in Au_NP (Fig. 2d). In order to further verify the degradation of ZnS_NP, the authors used Ellman’s reagent (DTNB) to detect the thiol ligands produced by the degradation of ZnS_NP. UV-visible absorption spectrum data confirmed that ZnS_NP degraded in aqueous solution and produced thiol ligands (Fig. 2e). The authors also verified the generation of sulfhydryl ligands using electrospray ionization-mass spectrometry (ESI-MS) (see the supplementary material). These results indicate that ZnS_NP has good degradation properties.

Figure 2. Comparison of degradation properties of ZnS_NP and Au_NP
Next, the authors loaded ruthenium metal catalysts on the surfaces of ZnS_NP and Au_NP respectively to form ZnSand Au_NZ. The results of the uncaging reaction of rhodamine fluorescent dye precursor molecules show that the catalytic activity of ZnSis approximately 2.5 times higher than that of Au_NZ (Figure 3a). ZnShas higher maximum reaction rate and substrate affinity than Au_NZ (Fig. 3b–c). In the decaging reaction of the rhodamine fluorescent dye precursor molecule, the transfer of the vinyl group from the ruthenium catalyst to the nucleophile is the rate-determining step of the entire decaging reaction and determines the overall efficiency of the decaging reaction (Figure 3d). Therefore, nucleophiles in the reaction system play an important role in increasing the reaction rate. The authors speculate that the sulfhydryl ligand released during the degradation of ZnShas strong nucleophile ability and can serve as a nucleophile to accelerate the uncaging reaction. The results of ESI-MS confirmed this conjecture. The signal peak at the charge-to-mass ratio of 462 is the strongest, which is the product after the vinyl group is transferred to the thiol ligand, while the signal peak at the charge-to-mass ratio of 421 is very weak. This shows that most thiol ligands react with vinyl groups rather than forming disulfide compounds through oxidation reactions (Figure 3e).

Figure 3. Catalytic activity characterization and mechanism study of ZnSand Au_NZ
On this basis, the author used ZnSto catalyze the uncaging reaction of rhodamine fluorescent dye precursor molecules in living cells. The uncaged rhodamine molecules have a green fluorescent signal. As shown in the confocal fluorescence microscopy imaging results in Figures 4a and 4b, the intracellular fluorescence intensity of the ZnSgroup is significantly stronger than that of the Au_NZ group, indicating that ZnShas higher catalytic activity, which is consistent with the activity characterization results in aqueous solution. The characterization results of flow cytometry also further proved that the intracellular fluorescence intensity of the ZnSgroup was significantly stronger than that of the Au_NZ group (Figure 4c). These results indicate that ZnScan efficiently catalyze the uncaging reaction in living cells.

Figure 4. Characterization of the uncaging reaction of rhodamine precursor molecules catalyzed by ZnSand Au_NZ in living cells.
Finally, the authors applied ZnSfor the activation of anticancer prodrugs in living cells. Mitoxantrone prodrug (pro-Mit) has very weak cytotoxicity, but after removing the allyloxycarbonyl protecting group through a uncaging reaction, it becomes an anticancer drug with strong cytotoxicity (Figure 5a). As shown in Figure 5b, thanks to the higher catalytic activity of the uncaging reaction, ZnScan induce stronger cytotoxicity than Au_NZ at the same dose. Therefore, in practical applications, ZnSnanozymes with higher catalytic activity require less dosage, which can reduce their potential toxic side effects and have better application prospects.

Figure 5. ZnSand Au_NZ are applied to the activation of anticancer prodrugs in living cells.
Supplier
TRUNNANOÂ is a supplier of Zinc Sulfide with over 12 years experience in nano-building energy conservation and nanotechnology development. It accepts payment via Credit Card, T/T, West Union and Paypal. Trunnano will ship the goods to customers overseas through FedEx, DHL, by air, or by sea. If you are looking for high-quality Zinc Sulfide, please feel free to contact us and send an inquiry.
Â